Author:-
Mr.Nirav RajendraKumar Soni, A-one pharmacy College,Anasan , Ahmadabad ,Gujarat ,India,Contact Number :9033002567
Download Guest Post
Download Guest Post
| Abstract : |
Gliclazide contains not less than 99.0 per cent and not more than the equivalent of 101.0 per cent of 1-(hexahydrocyclopenta[c]pyrrol-2(1H)-yl)-3-[(4- methylphenyl)sulphonyl]urea, The primary mechanism of action of gliclazide in lowering blood glucose appears to be dependent on stimulating the release of insulin from functioning pancreatic beta cells. Gliclazide has been shown have extra pancreatic effects like reduction in platelet adhesiveness and aggregation and increase in fibrinolytic activity.Minimize dosing frequency , Minimizes fluctuations in serum drug levels ,For a drug having narrow therapeutic index, To achieve zero order release rate of drug ,Patients compliance.In preparation of Gliclazide having ingredients CHPD,Mg-Sterate , Colloidal Silica ,HPMC and Maltodextrin compatible with each other .In this preparation Gliclazide SR -30 mg having less cost effective and Intended for single dose administration per day & better patient compliance with sharping effect against diabetic condition.
Key word :
· Ingredients
· Preformulation study
· Process parameter
· Formulation parameter
· In vitro drug release study and stability study
· QTPP(Quality target product profile )
Introduction :
Ideally a drug to provide desired therapeutic action should arrive rapidly at the site of action in optimum concentration, remain there for the desire time, be excluded from other site and get rapidly removed from the site of planned after its action. The fact that absorption rate of drug into the body can be decreased by reduction of the rate of release of the drug from the dosage form is one of the most recent and interesting result of pharmaceutical research. This ideal dosing regimen, which enhances patient compliance and helps guard against overdosing and side effects, is made possible by controlled release delivery systems, which use a variety of mechanisms to deliver and maintain the drug at a certain level in the patient’s blood stream1 This ideal dosing regimen, which enhances patient compliance and helps guard against overdosing and side effects, is made possible by controlled release delivery systems, which use a variety of mechanisms to deliver and maintain the drug at a certain level in the patient’s blood stream2
Gliclazide is a second-generation hypoglycemic sulfonylurea that is useful in the treatment of Type 2 diabetes mellitus . Gliclazide shows good tolerability and a low incidence of hypoglycemia; a low rate of secondary failure inhibits platelet aggregation and increases fibrinolysis . Thus gliclazide appears to be a drug of choice in long-term sulfonylurea therapy for the control of Type 2 diabetes mellitus . It shows low aqueous solubility and dissolution rate and often shows low and irregular bioavailability after oral administration. The enhancement of oral bioavailability of poorly watersoluble drugs remains one of the most challenging aspects of formulation development. The solid dispersion of poorly water-soluble drugs in water-soluble polymers enhances drug dissolution and bioavailability.The preparation and characterization of complexes of gliclazide with β-cyclodextrin have been reported . Complexation of gliclazide with β-cyclodextrinhydroxypropyl methylcellulose, which enhanced its hypoglycemic activity, has been reported . In addition, accelerated absorption of gliclazide using PEG 400 was studied earlier . Solid dispersions of gliclazide in PEG 6000 have been developed to increase drug dissolution rate . Enhancement of the solubility of gliclazide using polyvinylpyrrolidone K90 has been reported . The molecular weight of the polymer may play a role in the performance of a solid dispersion. The rationale of the present study was to investigate the use of lower molecular weight PEG 4000 for the preparation of solid dispersions with the objectives of improving dissolution. The solubility and dissolution rate of gliclazide can be enhanced in SDs with PEG 4000. The solubilization effect of PEG 4000 rate of gliclazide and obtaining different behavior as compared with PEG 60003-6
Sustained Release Drug Delivery System
Oral drug delivery has been known for decades as the most widely utilized route of administration among all the routes that have been explored for the systemic delivery of drugs via various pharmaceutical products of different dosage forms. The reasons that the oral route achieved such popularity may be in part attributed to its ease of administration as well as the traditional belief that by oral administration the drug is as well absorbed as the food stuffs that are ingested daily. In fact the development of a pharmaceutical product for oral delivery, irrespective of its physical form involves varying extents of optimization of dosage form characteristics within the inherent constraints of GI physiology. Therefore a fundamental understanding of various disciplines, including GI physiology, pharmacokinetics, pharmacodynamics and formulation design are essential to achieve a systemic approach to the successful development of an oral pharmaceutical dosage form. The more sophisticated a delivery system, the greater is the complexity of these various disciplines involved in the design and optimization of the system. In any case, the scientific framework required for the successful development of an oral drug delivery system consists of a basic understanding of the following three aspects;Physicochemical, pharmacokinetic and pharmacodynamic characteristics of the drug.The anatomic and physiologic characteristics of the GIT.Physicochemical characteristics and the drug delivery mode of the dosage form to be designed.7Oral ingestion has long been the most convenient and commonly employed route of drug delivery. Indeed, for sustained-release systems, the oral route of administration has by far received the most attention with respect to research on physiological and drug constraints as well as design and testing of products. This is because there is more flexibility in dosage form design for the oral route than there is for the parenteral route.8 The goal in designing sustained or controlled-delivery systems is to reduce the frequency of dosing or to increase effectiveness of the drug by localization at the site of action, reducing the dose required, or providing uniform drug delivery9.The enormous problems of patient compliance as well as the therapeutic desirability of controlled tissue drug levels over the time course of therapy are sufficiently compelling reasons to warrant placement of drugs in a sustained form of drug delivery10 In the past, many of the terms used to refer to therapeutic systems of controlled and sustained release have been used in an inconsistent and confusing manner. Sustained release, sustained action, prolonged action, controlled release, extended action, timed release, depot, and repository dosage forms are terms used to identify drug delivery systems that are designed to achieve a prolonged therapeutic effect by continuously releasing medication over an extended period of time after administration of a single dose11 It is an anti-daiabetic containg drug involved sulphonylureas having class mentioned below:
CATEGORY: It is a sulphonylureas
USE: antidiabetic (in NIDDM-Non insulin Dependent Diabetes mellitus )
HALF LIFE: 10.4 h (Duration of action is 10-24 h
BCS CLASS: class II
DOSE: 40-320 mg (in two divided dose) 30 & 60 mg(for MR)
Field of the invention:
The present invention relates to matrix tablet that enables the prolonged release of gliclazide ,the release being insensitive to variation in the pH of the dissolution medium,and that ensures regular and continuous blood levels after absorption of the galenic form by ORAL ROUTE
By Matrix tablet invention :
The controlled release is linear for a period of more than 8 Hrs and is such that 50% of the total amount of gliclazide has been release between 4 to 6 Hours after administration moreover
The Matrix tablet according to the invention enables prolonged release of gliclazide that results in Humans in blood levels of from 400 to 700 ng/ml 12 Hours at most after a single administration by the oral route of a tablet containing a dose of 30 mg gliclazide , and in blood levels of from 250 to 1000 ng/ml after a daily administration of a tablet containing a dose of 30 mg of gliclazide12-15.
INGREDIENTS:
Ingredient | Role | Justification |
Glicalazide | API (active pharmaceutical ingredient) | Anti-diabitic action |
Calcium hydrogen phosphate dehydrate (CHPD) | Enables improved granule fluidity and granule compressibility Also slow down the dissolution kinetics . To control the dissolution profile of the active ingredient | In the final blend •Compatible with API, Particle size needs control |
Magnesium Sterate (0.25-4%) | Lubricant | • In the final blend. • Vegetable origin •Compatible with API • Particle size needs control |
Colloidal silica | Flowing agent | Enhance flow property Compatible with API |
Hydroxyl propyl methyl cellulose (HPMC) Matrix forming polymer (5-20 % ) | Granulation purpose To control dissolution profile | Particle size needs control . Compatible with API |
Maltodextrine (0.25- 3 %) | Enabling release of active ingredient that is perfectly prolonged and controlled | Granulation Compatible with API |
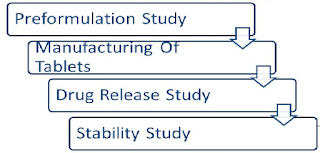
Preformulation Study :
Ø Solubility Enhancement of GLICLAZIDE16
Ø Method: Solvent Melting Method
Ø Carrier: PEG 6000
Ø Before: 40 % drug release/1 min
Ø After :100% drug release/1 min
Profile Component | Method | Criteria |
Flow Property | Angle of repose: Inversly prop. Carr’s index: inversly prop, Hausner ratio, | <30 Ф |
Solubility | In Water | 1-10mg/ml |
Melting point |
| - |
Stability (With all excipients to be used) | FTIR Spectroscopy ,DSC | Must be Compatible |
Assay | UV (227 nm) | 95-105% |
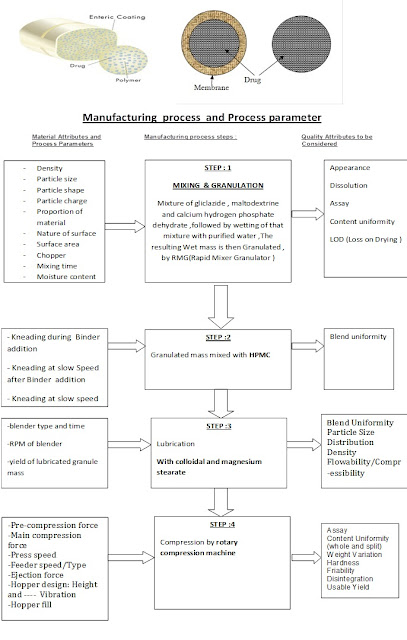
PROCESS & PARAMETER:-
MANUFACURING PROCESS FLOW
In vitro Drug Release Study :
· Dissolution parameter:
Ø Medium: 0.1N Hydrochloric acid followed by 6.8 Phosphate buffer
Ø Volume: 900ml
Ø Apparatus: USP-I (Basket)
Ø RPM: 50 rpm
Ø Temperature: 37°C ± 0.5°C
Ø Time 24 hrs
Comparision with Market Preparation(Similarity study)
Stability Study:
Ø Acelarated Stability syudy:
Ø Tablets stored for 3 months 40.2 oC and 75.5% RH.
Ø Carry out DSC for excipient compatibility
Ø Carry out in-vitro dissolution study and compare it with before 3 months results
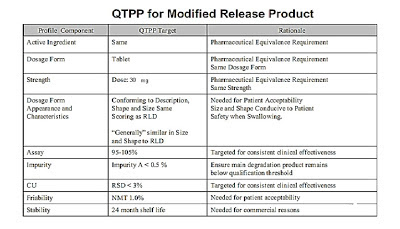
Quality Target Product Profile
Summary :
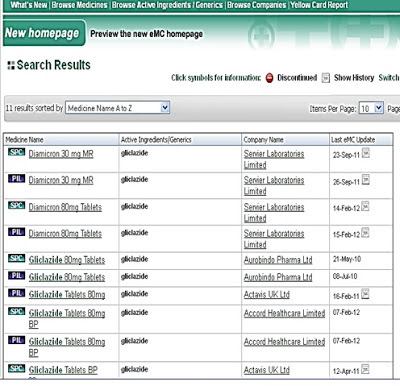
Diamicron 30 mg MR (Available Products in Regulated Marketed)
Ø Summary product characteristics emc (Electronic medicine compendium)
Ø It is marketed as Glizid, Glyloc and Reclide in India &
Ø Diamicron in Canada. In the Philippines, Servier markets it as Diamicron MR
Ø Many generic equivalents are also available e.g. Glubitor-OD, Clizid. It is not marketed in the United States (US).
Result and Conclusion :For above preparation of GLICLAZIDE SR-30 mg is better compliance of a patient and also reducing cost , and Intended for single dose administration per day Giving sharping effect against diabetic condition. Solubility enhanced by PEG -6000 and/or Beta –cyclodextrin . According to our preparation having more effective to turn against diabetes condition .
References :
1. Asgar A, Sharma SN. Evaluation of oral sustained release formulation. The East
Pharm. 1991:69-74.
2. Haider SS, Monnujan N, Shahriyar SM. Sustained release preparation of
metoclopramide hydrochloride based on fatty matrix. Indian Drugs 2002; 39:73-9.
3. Reynolds, J. E. F., Ed. Martindale: The Extra Pharmacopoiea XXX, 30th ed.;
Pharmaceutical Press: London, 1993; pp 279–280.
4 . Dollery, S. C., Ed. Therapeutic Drugs; Churchill Livingstone: London, 1991.
5 . Harrower, A. D. Comparison of efficacy, secondary failure rate and
complications of sulfonylurea. J. Diabetes Complicat. 1994, 8, 201–203.
6 . Palmer, K. J.; Brogden, R. N. Gliclazide, an update of its pharmacological properties
and therapeutic efficacy in NIDDM. Drugs 1993, 46, 92–125.
7. Yie WC. Novel Drug Delivery Systems. 2nd ed. New York: Marcel Dekker Inc; 1992
8 . Robinson JR, Lee V. Controlled Drug Delivery Fundamentals and Applications. 2nd ed.
New York: Marcel Dekker Inc; 373-4 .
9. Banker GS, Rhodes CT. Modern Pharmaceutics. 3rd ed. New York: Marcel Dekker
Inc. 1996; 575-609.
10. Gudsoorkar VR, Rambhau D. Sustained release of drugs. The East Pharm. 1993; 36:17-22.
11 Lachman L, Lieberman HA, Kanig JL. The Theory and Practice of Industrial Pharmacy.
3rd ed. Mumbai: Varghese Publishing House. 1987. p. 293-345.
12. Vyas SP, Khar RK. Controlled Drug Delivery: Concepts and Advances.
1st ed. Delhi: Vallabh Prakashan; 2002
13 . Alderman DA. Swellable matrices as systems for oral delivery. Int J Pharm. 1984; 1-5.
14 Nigayale AG. Investigation of prolonged drug release from matrix formulation of
chitosan. Drug Dev Ind Pharm. 1990; 16: 449-67.
15. Gomez AD. Role of water-uptake on tablet disintegration: design of improved
method for penetration measurements, Acta Helv. 1986, 61, 22-29
16. S. Biswal, ‘Enhancement of Dissolution Rate of Gliclazide Using Solid Dispersions




0 comments:
Post a Comment