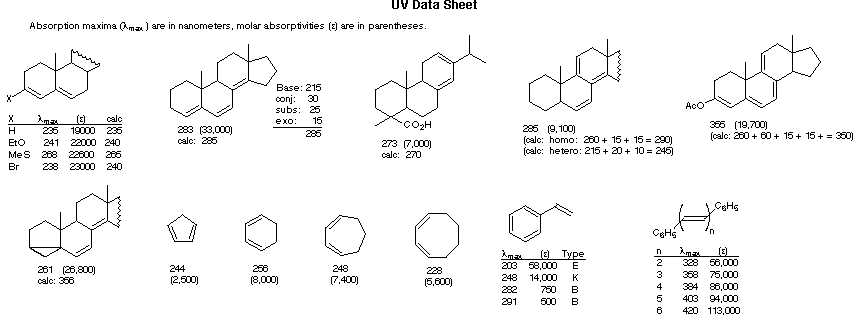
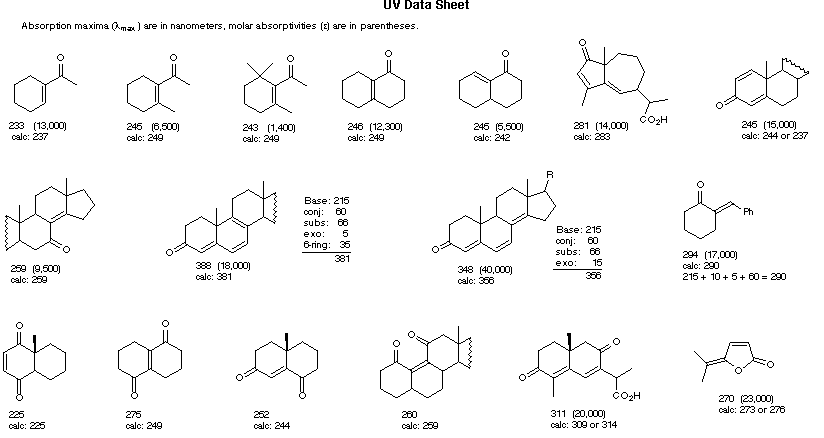
Empirical Rules for Absorption Wavelengths of Conjugated Systems
Core Chromophore | Substituent and Influence |
|---|---|
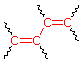 Transoid Diene 215 nm | R- (Alkyl Group) .... +5 nm RO- (Alkoxy Group) .. +6 X- (Cl- or Br-) ......... +10 RCO2- (Acyl Group) .... 0 RS- (Sulfide Group) .. +30 R2N- (Amino Group) .. +60 C6H5 (Phenyl Group) ... +60 |
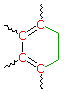 Cyclohexadiene* 260 nm | |
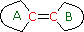 (i) Each exocyclic double bond adds 5 nm. In the example on the right, there are two exo-double bond components: one to ring A and the other to ring B. (i) Each exocyclic double bond adds 5 nm. In the example on the right, there are two exo-double bond components: one to ring A and the other to ring B. (ii) Solvent effects are minor. * When a homoannular (same ring) cyclohexadiene chromophore is present, a base value of 260 nm should be chosen. This includes the ring substituents. Rings of other size have a lesser influence. | |
λmax (calculated) = Base (215 or 260) + Substituent Contributions
Some examples that illustrate these rules follow.
Core Chromophore | Substituent and Influence |
|---|---|
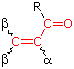 R = Alkyl 215 nm R = Alkyl 215 nmR = H 210 nm R = OR' 195 nm | α- Substituent R- (Alkyl Group) +10 nm Cl- (Chloro Group) +15 Br- (Chloro Group) +25 HO- (Hydroxyl Group) +35 RO- (Alkoxyl Group) +35 RCO2- (Acyl Group) +6 β- Substituent R- (Alkyl Group) +12 nm Cl- (Chloro Group) +12 Br- (Chloro Group) +30 HO- (Hydroxyl Group) +30 RO- (Alkoxyl Group) +30 RCO2- (Acyl Group) +6 RS- (Sulfide Group) +85 R2N- (Amino Group) +95 γ & δ- Substituents R- (Alkyl Group) +18 nm (both γ & δ) HO- (Hydroxyl Group) +50 nm (γ) RO- (Alkoxyl Group) +30 nm (γ) C6H5 (Phenyl Group) ... +60 |
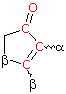 Cyclopentenone 202 nm | |
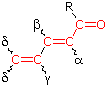 | |
 (i) Each exocyclic double bond adds 5 nm. In the example on the right, there are two exo-double bond components: one to ring A and the other to ring B. (i) Each exocyclic double bond adds 5 nm. In the example on the right, there are two exo-double bond components: one to ring A and the other to ring B. (ii) Homoannular cyclohexadiene component adds +35 nm (ring atoms must be counted separately as substituents) (iii) Solvent Correction: water = –8; methanol/ethanol = 0; ether = +7; hexane/cyclohexane = +11 | |
λmax (calculated) = Base + Substituent Contributions and Corrections
Some examples that illustrate these rules follow.




0 comments:
Post a Comment